Comments regarding laboratory methods
Platelet-binding activity
The clinical utility of traditional ristocetin cofactor (VWF:RCo) assay is compromised by the large assay variability, suboptimal sensitivity and high VWF detection limit. Alternative assays of VWF:RCo may overcome these disadvantages. Assays developed in recent years measure binding to GPIb, or fragments thereof, directly, or indirectly though specific antibodies and with or without ristocetin as modulating agent.
Nomenclature for VWF activity assay methodologies has been established as follow: 1) assays based on fixed platelets and platelet agglutination in the presence of ristocetin has the old name VWF:RCo, 2) assays based on recombinant GPIb and ristocetin are termed VWF:GPIbR, and 3) assays based on the GPIb containing gain-of-function mutations are termed VWF:GPIbM [1,2].
The Nordic Hemophilia Council recommends the new VWF platelet-dependent VWF activity assays (VWF:GPIbR, VWF:GPIbM) over the VWF:RCo. Please note that the use of “VWF:RCo” is still widely used in scientific literature, and it is used in these guidelines, to describe VWF platelet-binding activity in general. Much of the data presented here is also based on results that were produced before the newer activity methods came into use. Thus, when VWF:RCo is stated in these guidelines, it refers to VWF platelet-binding activity without further specification of which activity assay was used.
The VWF:GPIbR involves binding of active plasma VWF, in the presence of ristocetin, to a recombinant fragment of wild-type GPIb coated onto latex particles through a monoclonal antibody. The particles agglutinate with a decreased light transmission, which is directly proportional to the ristocetin-mediated plasma VWF:GPIb-binding activity [3]. There is also a variant of this assay based on magnetic particles with chemiluminescent technology [4].
The VWF:GPIbM utilizes a recombinant GPIbα fragment containing two gain-of-function mutations (G233V, M239V), which bind plasma VWF via the GPIb receptor in the absence of ristocetin and shear stress. Added latex particles coated with an antibody against GPIb will bind the VWF-recombinant GPIbα complex, inducing microparticle agglutination and decreased light transmission, which is directly proportional to the VWF:GPIb-binding activity in the plasma [5,6]. The gain-of-function mutations introduced into the GPIb fragments originate from the platelet type or pseudo VWD, which are characterized by spontaneous binding of VWF to platelets carrying the mutant GPIb, hence there is no requirement for ristocetin [7].
Von Willebrand factor antigen (VWF:Ag)
The concentration of VWF in plasma is measured with immune assays. Common methods are based on the ELISA technique or using an automated latex-enhanced immunoturbidometric assays performed with coagulation analyzers. There is also an automated commercially available chemiluminiscent assay. The assay principles have similar diagnostic capacity, but the reproducibility is better using automated assays [8].
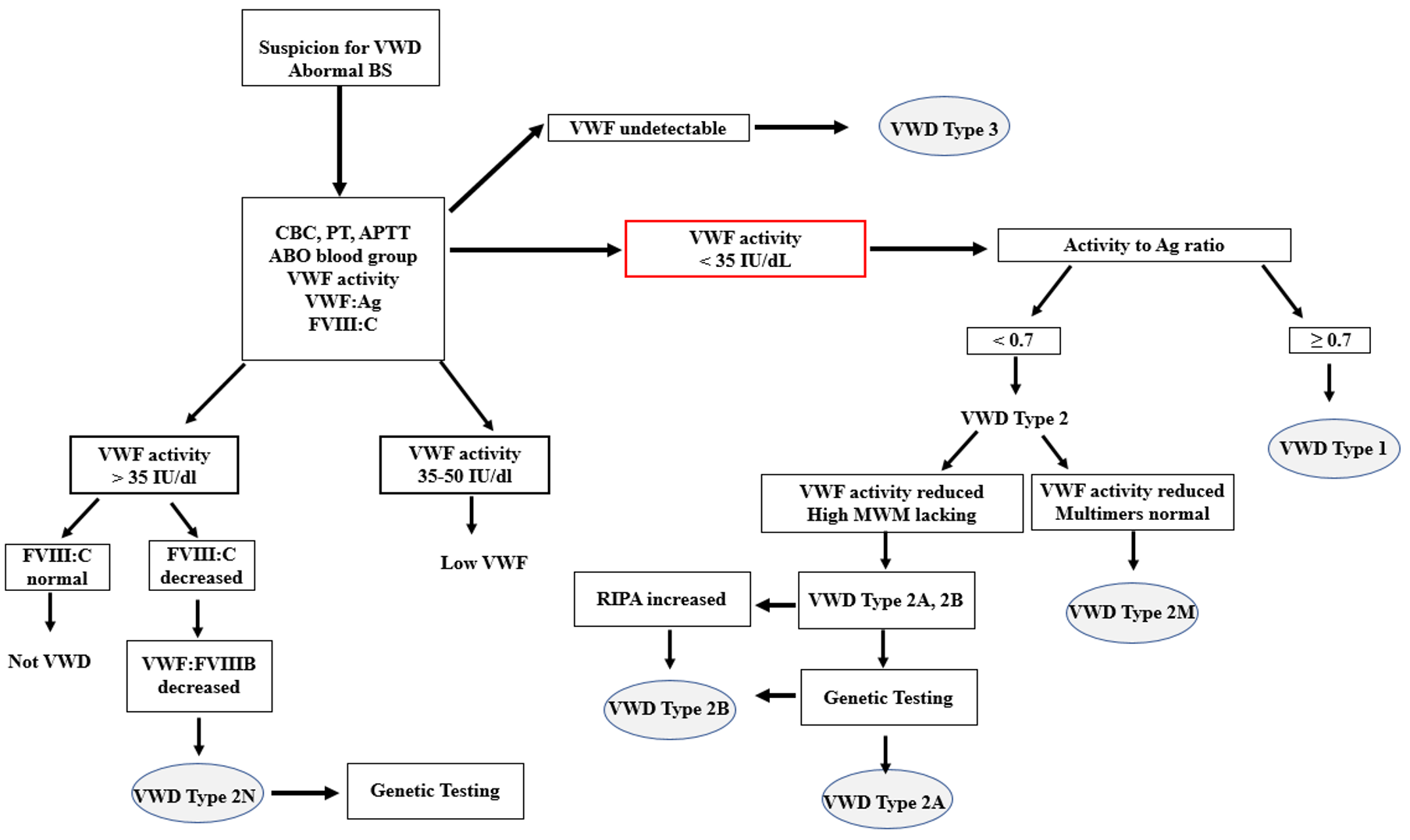
Normal plasma concentrations of VWF:Ag may be found in patients having VWD due to qualitative defects in VWF. For example, most type 2N (if not compound heterozygous) and some patients with other type 2 variants may express quite normal quantities of antigen, which is, however, dysfunctional. Thus, a reduced ratio between VWF activity/VWF:Ag may indicate a VWD type 2 phenotype. A cut off <0.7 has been recommended for platelet-dependent VWF activity/VWF:Ag in order to suspect VWD type 2A, 2B or 2M [9]. Similarly, a reduced ratio between FVIII:C/VWF:Ag may be used to identify patients with VWD type 2N.
Von Willebrand factor collagen binding (VWF:CB)
The method relies on the ability of VWF to adhere to collagen and is usually performed as an ELISA, but automated assays are available [10,11]. The assay principle utilizes collagen as a natural ligand to VWF, immobilized either on the plastic surface of an ELISA-plate or on the surface of very small plastic and magnetic particles. After incubation with plasma and washing, the bound VWF is detected using an enzyme-conjugated anti-VWF antibody, and a specific substrate will provide a color or chemiluminescent signal that is proportional to the VWF collagen binding capacity. The source and type of collagen used in the assay may differ between different manufacturers, which also have different collagen formulations in their kits. In general, collagen type I or type III, or a combination of the two, is used. One commercially available reagent utilizes a synthetic triple-helical peptide of collagen type III [4,11].
The method reflects a biological function of VWF and contributes to the diagnosis of VWD by providing information about the quality of VWF. The VWF:CB and the platelet-dependent VWF:GPIbR or VWF:GPIbM activity assays often show positive correlation with the multimeric structure of VWF and are used to classify VWD type 2 variants. The type 2M subtype is identified as reduced VWF activity, usually the platelet-dependent activity, together with normal multimeric pattern. However, variants with selectively low VWF:CB (and normal platelet-dependent activity) have also been described [12]. Thus, type 2M could be further divided into type 2MGPIb and type 2MCB, depending on the phenotype with the VWF activity assays. This also indicates that the VWF:CB assay should not replace the VWF:GPIbR or VWF:GPIbM assays but rather used as a supplementary assay. The approach to combine a platelet-dependent activity assay, and the VWF:CB assay has been shown to reduce diagnostic errors in clinical laboratories [4].
Multimeric sizing electrophoresis techniques, VWF:Multimers
The assay evaluates the distribution of VWF molecules with different molecular weights (multimers). The study of VWF multimer composition in plasma is based on electrophoresis in a gel system suited for separation of macromolecules. Following separation, VWF molecules are electroeluted onto an immobilizing membrane where patterns of multimeric subsets are identified by means of an immuno-enzymatic or lumographic technique. The test is complex, cumbersome, and difficult to perform and interpret. Therefore, it should be performed only in specialized laboratories with long-term experience. However, in recent years, a CE-marked commercial standardized system with pre-casted gels has been available. The system is semi-automated, results are obtained within one day and it has been validated for VWD diagnostics [13–15].
The method is most often qualitative (i.e., visual inspection of the multimer pattern) but quantification by integration of the area under a densitometric curve is possible which makes the interpretation of results easier. It is also possible to adapt the method by changing the gel system to focus on the large multimers or to increase the resolution in the low range, which may resolve abnormalities of triplet structure of each multimer [16]. The commercially available assay, with pre-casted gels, is developed for multimer analysis at low resolution and therefore cannot be used to study aberrations of the triplet structure and other band abnormalities.
In VWD type 1 all multimers are present, whereas VWD type 3 is characterized by loss of all multimers. VWD type 2A is often associated with a severe loss of large and intermediate multimers. Most type 2B patients display a loss of large multimers but exceptions, with normal multimers have been reported. Patients with VWD type 2M demonstrate all multimers, sometimes larger than normal (supranormal) or with bands resulting in a blurred (“smeary”) appearance. The multimers may also lack satellite bands in some type 2M samples. An aberrant multimer pattern may also be observed in type 2N due to abnormal disulphide bonds in the VWF molecule but the method cannot be diagnostically used in this subtype.
Von Willebrand factor binding to FVIII (VWF:FVIIIB)
The VWF:FVIIIB is an important test for correct classification of VWD type 2N (Normandy), and it is able differentiate between type 2N VWD and hemophilia A. In principle, in the assay patient’s VWF is bound to an ELISA microtiter plate and incubated with highly purified FVIII. After extraction, the bound fraction of F VIII:C, if present, is then determined. An absent, or very low, FVIII binding activity is indicative for VWD type 2N. The commonly performed VWF:FVIIIB assays comprise validated homemade assays but recently a commercially and CE-marked assay has become available.
The current ASH/ISTH/NHF/WFH guidelines suggest using either the VWF:FVIIIB or targeted genetic testing for patients with suspected VWD type 2N [9]. The indication for the assay is when coagulation assessment reveals a low FVIII level in a patient with a negative family history of hemophilia. The low VWF may be due to a decreased carrier effect of VWF. VWD type 2 N is inherited as a recessive trait and patients are either homozygous or compound heterozygotes for different type 2N mutations. Usually, the type 2N patients have FVIII:C values <40 IU/dL, associated with normal or reduced VWF levels but a low FVIII:C/VWF:Ag ratio.
Ristocetin-induced platelet aggregation (VWF:RIPA)
This test determines platelet aggregation as recorded in patient’s platelet-rich plasma (PRP) in the presence of ristocetin, using an aggregometer instrument. Aggregation occurs over a range of ristocetin concentrations and results from the ristocetin-induced interaction between VWF and platelet receptor GPIb complex. As the RIPA needs to be performed on fresh platelets it is necessary to perform the test within 2 h of blood collection.
This method is relatively insensitive to quantitative deficiencies of VWF. The RIPA is absent in type 3 VWD and generally decreased in type 2A VWD. In type 1 VWD the RIPA will depend on the concentration of VWF in plasma, with a reduced RIPA at very low concentration of VWF.
In contrast, type 2B variants display increased platelet aggregation to low concentrations of ristocetin. There is consensus that increased sensitivity to ristocetin concentration of 0.5 mg/ml or lower indicates the presence of type 2B VWD. Normal individuals will show in general platelet aggregation at and above 0.75 -1.0 mg/ml of ristocetin, but typically not below this concentration. In type 2B, this methodology is not well standardized, but usually ristocetin concentrations of 0.6 mg/ml or lower indicate a positive test result. Targeted genetic testing is recommended to confirm type 2B VWD diagnosis (see [Genotyping VWD] section).
Von Willebrand factor propeptide (VWFpp)
The VWF propeptide separates from the VWF precursor after VWF is secreted to the circulation. The propeptide is a large (100 kDa) glycopeptide and can be measured immunologically using the ELISA technique with a specific antibody.
The propeptide is important for correct dimerization and multimerization of VWF that will be secreted. However, it has no known function in the circulation. The VWFpp method represents a tool for identifying patients with acquired von Willebrand syndrome but also to characterize VWD types with shortened VWF half-life in plasma (increased clearance) [17–19]. In such cases, the ratio of VWFpp/VWF:Ag is high, as VWF, unlike the propeptide, is cleared rapidly. Thus, the VWFpp can be useful to differentiate between severe VWD type 1, i.e., type 1C, from type 3 and to distinguish specific VWD type 1 variants [19]. Furthermore, identifying increased clearance of VWF is of value when considering desmopressin treatment.
Anti-VWF antibodies
Alloantibody formation against VWF is rare, but may be detected in patients with VWD type 3 that have been treated with VWF-containing concentrates [20]. Autoantibodies to VWF can sometimes be associated with acquired von Willebrand syndrome.
The presence of allo- or autoantibodies that neutralize the VWF activity can be tested and quantified based on the same principle as anti-FVIII antibodies (i.e., Bethesda assay). In brief, the patient plasma is mixed with pooled normal plasma and then the VWF activity is measured and compared with a control of pooled normal plasma mixed with VWF-deficient plasma. As the antibodies may be directed against different parts of the VWF molecule, mixing experiments should include different activity tests, i.e., which examine the ability to bind GPIb and collagen. However, detecting neutralizing antibodies in patients who have normal/subnormal levels of VWF activity is difficult with the conventional Bethesda assay. A variant of the assay that better considers endogenous VWF activity in the sample was described by Mannucci et al. [21], which means that the patient plasma is mixed with normal plasma while two control samples are analysed, where the two control samples together give the “expected” value in the sample. Antibodies can be also detected by a so-called ELISA-based method, which involves immunological detection of anti-VWF antibodies. The anti-VWF ELISA detects all types of antibodies, both neutralizing and non-neutralizing antibodies.
DDAVP challenge (biological response)
To ensure sufficient response for clinical use most patients should be administered a DDAVP test dose. Testing children is usually omitted until the age of 4 years. Depending on the mode of administration and suitability for the patient the recommended test dose is 0.3 µg/kg intravenously or subcutaneously, and, even intranasally, This testing profile is designed to assess the response to DDAVP by measuring VWF and FVIII levels pre-treatment (baseline) and at two time-points (1 and 4-hours) post-treatment. Post-treatment VWF and FVIII levels are evaluated against baseline levels to ensure the increase and sustained levels. DDAVP is usually effective in patients with type 1 VWD whose baseline VWF and FVIII levels are higher than 10 U/dL. A good response to DDAVP is defined as an increase of peak VWF and FVIII activity levels at least 3-fold over the baseline levels. A further blood sample collected after 4 hours is advisable to exclude patients having a short half-life of released VWF and/or FVIII following DDAVP stimulation. Type 1C (C for clearance) is characterized by a significant decrease (<30 %) of the 4 hours post-infusion from the peak VWF levels [22].